Connect with us
Published
12 months agoon
By
Tyler Shultz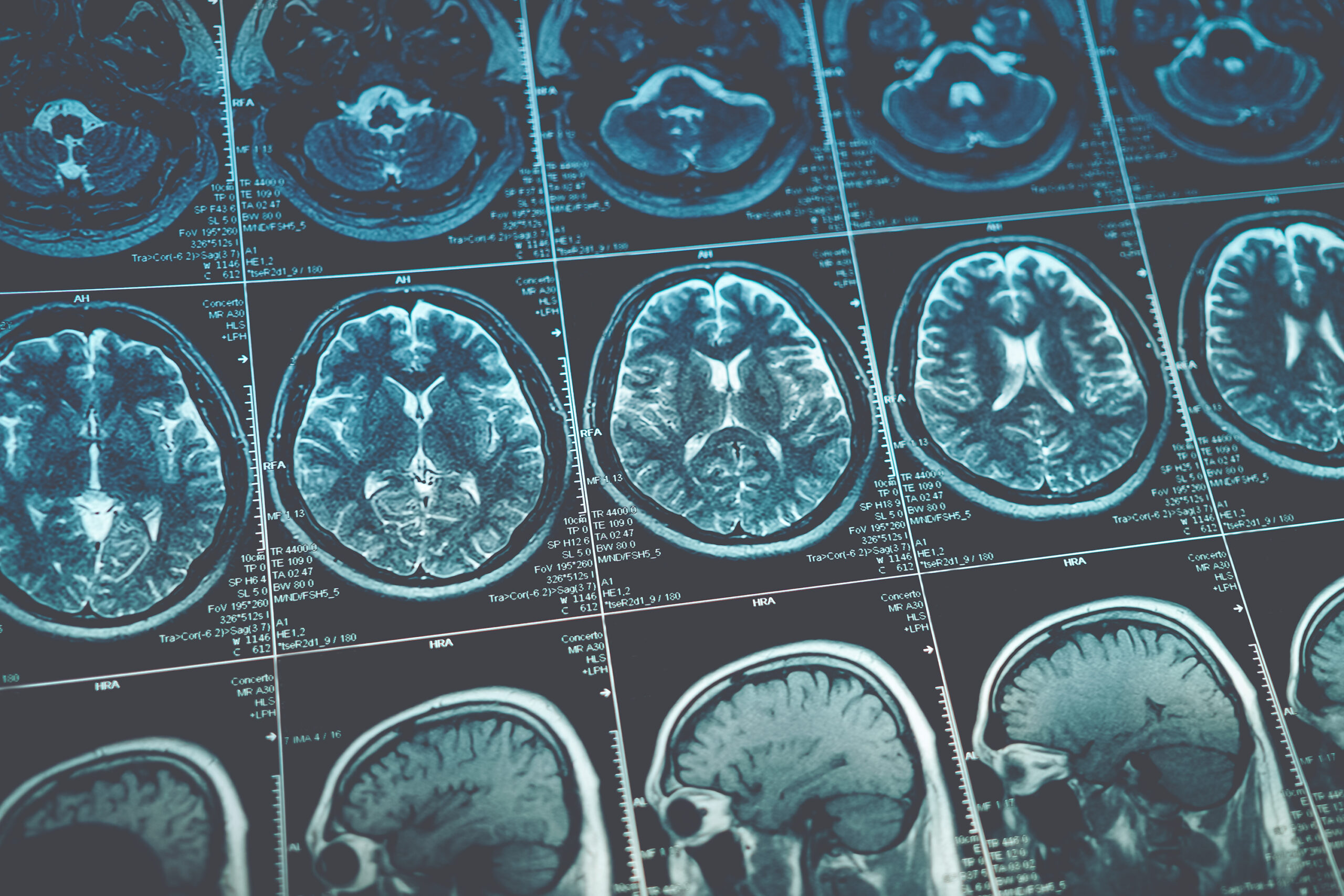
A grieving widow has welcomed a new trial that uses cannabis to treat glioblastoma, an incurable form of brain cancer.
Laura Smallbone, whose husband Peter passed away from glioblastoma after a 15-month battle with the disease, said she wants to see more options available for families like hers and expressed her excitement at the new trial.
“Pete was absolutely determined to make the most of what he had, and do all the fun things for as long as he could. Losing him bit-by-bit to this cruel disease was difficult, and I’d really like to see more options for families like ours. For 15 months we were asking ourselves ‘OK, so now what do we do?’ – there needs to be more treatment options for people diagnosed with glioblastoma. There also needs to be more awareness of the disease so there is a greater understanding of what families like ours had to face,” said Smallbone.
The ARISTOCRAT trial will assess if adding Sativex, an oral spray containing both THC and CBD already being used to treat multiple sclerosis, to existing chemotherapy treatment could extend the lives of patients diagnosed with glioblastoma. The trial hopes to recruit 230 patients at 15 National Health Service hospitals across all United Kingdom nations. During the first phase of the trial, researchers found 27 patients with recurring glioblastoma were able to tolerate chemotherapy and nabiximols, which contain a number of different cannabinoids.
“We now have the opportunity to take these laboratory results, and those from the phase one trial and investigate whether this drug could help glioblastoma patients live longer in this first-of-a-kind randomised clinical trial,” said Professor Short.
The three-year trial is led by Professor Susan Short at the University of Leeds and coordinated by the Cancer Research UK Clinical Trials Unit at the University of Birmingham. The new phase II trial is funded by The Brain Tumour Charity and experts hope Sativex can become one of the first additions to NHS treatment for glioblastoma since temozolomide chemotherapy in 2007.
“We know there has been significant interest among patients and researchers alike for some time about the potential activity of cannabinoids in treating glioblastomas. We’re really excited that this world-first trial here in the UK could help accelerate these answers and are so grateful to everyone who has donated to help us make this study possible – thank you,” said Dr David Jenkinson, Chief Scientific Officer at The Brain Tumour Charity. “The recent early-stage findings were really promising and we now look forward to understanding whether adding Sativex to chemotherapy could help offer life-extension and improved quality of life, which would be a major step forward in our ability to treat this devastating disease.”
Another study funded by The Brain Tumour Charity found medical cannabis can help alleviate cancer pain and reduce the number of drugs a patient needs. Over a three-and-a-half-year period, researchers studied 358 adults with cancer found products with an equal mixture of THC and CBD was the most effective treatment and resulted in significant reductions in average pain intensity, overall pain severity, and pain interference with daily life.
The study, published in BMJ Supportive & Palliative Care, said the data suggests medical cannabis may be “a safe and complementary treatment option in patients with cancer failing to reach adequate pain relief through conventional analgesics, such as opioids.”
A U.K parent with experience using medical cannabis for life-changing treatment has previously called for the wider use and availability of cannabis-based medicines. Hannah Deacon’s son, who previously suffered from around 500 seizures a day, recently celebrated his 1,000th seizure free day after taking Bedrolite, a CBD oil with less than 1% THC and 7.5% CBD. Deacon co-founded MedCann Support for families looking to access medical cannabis and has expressed frustration at the lack of action the National Institute for Health and Care Excellence Guidance


Despite City Efforts, Hemp Shops Posing as Dispensaries Prevail in Las Vegas

Cannabis Community, Investors React to DEA Decision To Reschedule


Georgia Governor Signs Bill Establishing Licensing Requirements To Grow Hemp


Study: Psilocybin Enhances Meditation


Ohio GOP Lawmakers Debate Adult-Use MJ Priorities, Eye June for Regulation Approval


Taylor Swift Puts Narcotics Into All of Her Songs on ‘The Tortured Poets Department’
